

The test protocols are traced to and from the requirements and specifications they verify. For Class II, III, (safety class C), the tracing needs to map the test case to the design element, design element to software requirement, software requirement to product requirement.
#GAMP SOFTWARE CATEGORIES VERIFICATION#
The (best practice) aim of conducting verification is to demonstrate that the system functions as intended, by using the requirements and specifications as an objective standard to which the system is tested. Therefore, the extent of testing performed would also be reduced. However, when working with commercial off-the-shelf software, (Category 3) then functional and configuration specifications are not required. For example, for a configured product (Category 4), then requirements, functional and configuration testing is conducted to verify the requirements. The types of specifications associated to a system are tied to its degree of complexity.
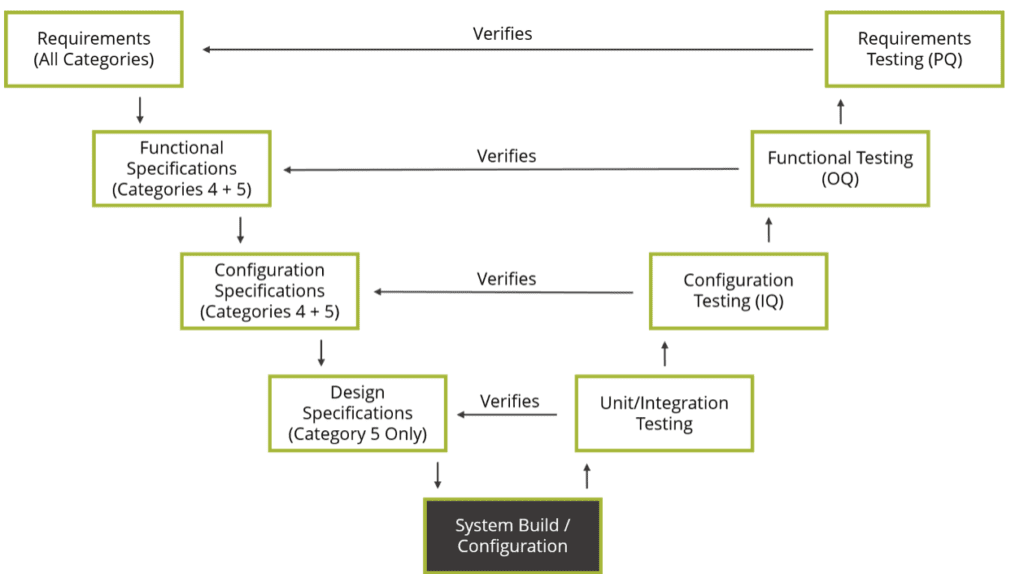
Traceability across the V results in tracing test cases to the design elements, design elements to software requirements, software requirements to product requirements. The V model juxtaposes the specifications produced for a system to the testing performed as part of the verification process. Specifications and design down the left side of the V and verification and validation up the right side. Let’s look at what GAMP 5 means, and what differentiates this guideline from other standards and regulations. Per Chrysa Plagiannos from Montrium, and the International Society for Pharmaceutical Engineering ( ISPE) document titled “ GAMP 5: A Risk-Based Approach to Compliant GxP Computerized Systems”, GAMP 5’s approach can be summed up by the V model diagram. GAMP is an acronym for Good Automated Manufacturing Practice, (which is hopefully quite familiar to you), for a risk-based approach to computer system validation where a system is evaluated and assigned to a predefined category based on its intended use and complexity.Ĭategorizing the system helps guide the efficient writing of system documentation (including specifications and test scripts and everything in between). Improved customer satisfaction with your productsįirst, let’s take a brief look at what GAMP 5 is and how it can benefit your company.


 0 kommentar(er)
0 kommentar(er)
